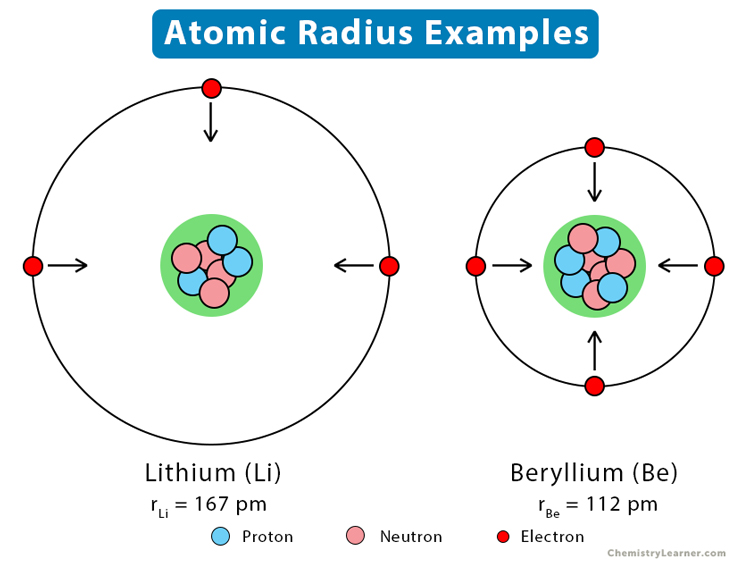
Atomic Radius And Why . Atomic radii can be obtained. Atomic and ionic radius increase moving down a group or column of the periodic table. This is because atoms gain an electron shell. periodic table trend. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together.
from www.chemistrylearner.com
Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: periodic table trend. Atomic and ionic radius increase moving down a group or column of the periodic table. Atomic radii can be obtained. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. This is because atoms gain an electron shell.
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table
Atomic Radius And Why This is because atoms gain an electron shell. periodic table trend. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Atomic and ionic radius increase moving down a group or column of the periodic table. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: Atomic radii can be obtained. This is because atoms gain an electron shell.
From slideplayer.com
TABLE PERIODIC. ppt download Atomic Radius And Why Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: Atomic and ionic radius increase moving down a group or column of the periodic table. periodic table trend. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. This is because atoms gain. Atomic Radius And Why.
From blog.prepscholar.com
Understanding Atomic Radius Trends The 2 Key Principles · PrepScholar Atomic Radius And Why This is because atoms gain an electron shell. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely. Atomic Radius And Why.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher Transition Metal Trend in atomic radius. Atomic Radius And Why Atomic radii can be obtained. periodic table trend. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic and. Atomic Radius And Why.
From slideplayer.com
ppt download Atomic Radius And Why the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. This is because atoms gain an electron shell. periodic table trend. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in. Atomic Radius And Why.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps Atomic Radius And Why Atomic and ionic radius increase moving down a group or column of the periodic table. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this. Atomic Radius And Why.
From elchoroukhost.net
Periodic Table Atomic Radius Elcho Table Atomic Radius And Why the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: atomic radius is the distance from the atom’s nucleus to the outermost electron orbital,. Atomic Radius And Why.
From www.slideserve.com
PPT Atoms PowerPoint Presentation, free download ID2682421 Atomic Radius And Why Atomic radii can be obtained. periodic table trend. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: Atomic and ionic radius increase moving down a group or column of the periodic table. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together.. Atomic Radius And Why.
From www.vrogue.co
Periodic Table Of The Elements Atomic Radius vrogue.co Atomic Radius And Why Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Atomic and ionic radius increase moving down a group or column of the periodic table.. Atomic Radius And Why.
From www.slideserve.com
PPT Section 4.5—Periodicity PowerPoint Presentation, free download Atomic Radius And Why atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. periodic table trend. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Electron configuration determines the organization of elements. Atomic Radius And Why.
From mavink.com
Atomic Radius Diagram Atomic Radius And Why periodic table trend. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. This is because atoms gain an electron. Atomic Radius And Why.
From www.pinterest.com
Atomic Radius across a Period Chemistry lessons, Chemistry Atomic Radius And Why Atomic radii can be obtained. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: atomic radius is the distance from the atom’s nucleus. Atomic Radius And Why.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Atomic Radius And Why This is because atoms gain an electron shell. Atomic radii can be obtained. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. periodic. Atomic Radius And Why.
From www.slideserve.com
PPT History PowerPoint Presentation, free download ID1587611 Atomic Radius And Why the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic. Atomic Radius And Why.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID8887607 Atomic Radius And Why Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: periodic table trend. Atomic radii can be obtained. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with. Atomic Radius And Why.
From www.ck12.org
Atomic Radius Example 2 ( Video ) Chemistry CK12 Foundation Atomic Radius And Why atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. Atomic radii can be obtained. Atomic and ionic radius increase moving. Atomic Radius And Why.
From www.animalia-life.club
Ionic Radius Diagram Atomic Radius And Why atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. This is because atoms gain an electron shell. Atomic radii can be obtained. periodic table trend. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic. Atomic Radius And Why.
From 88guru.com
Atomic RadiusAn Overview 88Guru Atomic Radius And Why the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. This is because atoms gain an electron shell. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic and ionic radius increase moving down. Atomic Radius And Why.
From www.youtube.com
L8 Why Atomic Radius increases down the group and decreases along the Atomic Radius And Why atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. This is because atoms gain an electron shell. periodic table trend. Atomic and ionic radius increase moving down a group or column of the periodic table. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic. Atomic Radius And Why.